Filter Integrity Tester: LT-5000 FLIT
“Integrity Assured, Intelligence Built In..!”
The LT-5000 FLIT Filter Integrity Tester is a compact, high-precision system designed for reliable filter integrity measurement with advanced security and automation features.
CComply with ASTM, FDA 21 CFR Part 11, USP 1207, NABL, CE, ISO 9001:2008, EMA ANNEX 11, ProdNx
Features
- Offers a wide range of integrity testing, compatible with all existing test methods.
- Integrated security framework offering password protection, permission control, hierarchical authority grading, and electronic signature support.
- Compatible with SAP, ERP communication with various protocols like Profinet, UDB, OPC etc.
- Dedicated development team for customization
Application
- Filter Integrity Testers play a vital role precisely in the food and pharmaceutical industries
- Product range includes:
- Capsule filter housings
- Industrial filter cartridges
- Membrane filtration systems
- Syringe filter
Technical Specifications:
| SPECIFICATIONS | MICRO LEAK DETECTION |
|---|---|
| Input power | 100 to 260 VAC, 50 Hz, 120W |
| Environmental Condition | Ambient Temperature: +5°C to +40°C Relative Humidity: 10 to 80% |
| Unit | m bar/kpa/pa |
| Test Scope | BP: 50 to 9000 mbar DF: up to 1000 ml/min WI: 0.01 to 150ml/min |
| Test Accuracy | 0.075% |
| Modes | Bubble Point Test, Diffusion Flow Test, Water Intrusion Test |
| Print Function | Compatible |
| Internal Memory | Stores upto 5 years of data |
| Test Report | In-built non-editable, printable PDF format |
| Display Screen | 7"/10" color touch screen - resistive/capacitive |
| Connectivity | Serial Ethernet & USB |
| Weight | 6.5 Kg |
- Tamperproof and Non-Editable Audit Trail Data Format
- The time stamp of the change of the parameter value and the user making the change
- The audit trail records the following details:
- User Creation
- User Login/ Logout
- Wrong attempts at login
- User Block / Unblock by Administrator
- Old value and new value of the parameter change
- The time stamp of each event
- Electronic signatures used to sign electronic records must be unique to the individual, verifiable, and protected from unauthorized use.
- There must be controls in place to ensure that electronic signatures are applied only by authorized signatories and that they cannot be tampered with
- Review of the reports on the HMI Screen for Production, Alarms, and Audit Trail.
- Storage limit can be interlocked in terms of the number of batches produced or % of the memory consumed.
- HMI offers basic connectivity for data exchange with Central SCADA / MES / ERP by following the means:
- Through OPC UA
- Through Data file transfer
- Through USB / SD Card Backup
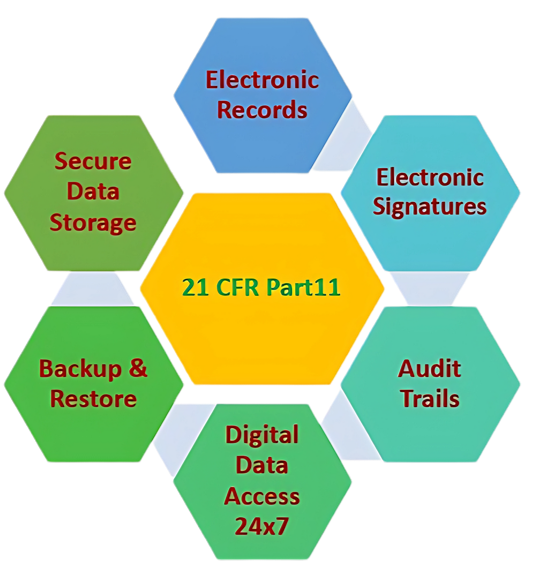
21 CFR PART 11 COMPLIANT LT-5000 FLIT
- To keep pace with the increasing use of electronic systems and technology in FDA-regulated industries, 21 CFR Part 11 was introduced, ensuring the same level of data integrity, authenticity, and reliability as paper-based systems.
- Basically, 21 CFR Part 11 is a regulation established by the U.S. Food and Drug Administration (FDA) that outlines the requirements for the use of electronic records and electronic signatures in FDA-regulated industries, including pharmaceuticals.
- Adhering to these guidelines, the introduction of Information Technology (IT) in compliance was a milestone.
- It establishes requirements for the security, integrity, and availability of electronic records and the validation of electronic systems used for GxP (Good Laboratory Practices, Good Clinical Practices, Good Manufacturing Practices) activities.
- Password-protected individual unique user account
- Password complexity
- Minimum 8 character password length
- Configurable number of wrong attempts
- User block/unblock facility
- Password validity
- Batch data is stored in a secure database format, and the Batch Report can be generated using this data.
- Report Printing: The report printing has been offered in multiple ways:
- Online printing of Alarms, Events, and Logged data through Serial Printer
- Printing of the General report through USB
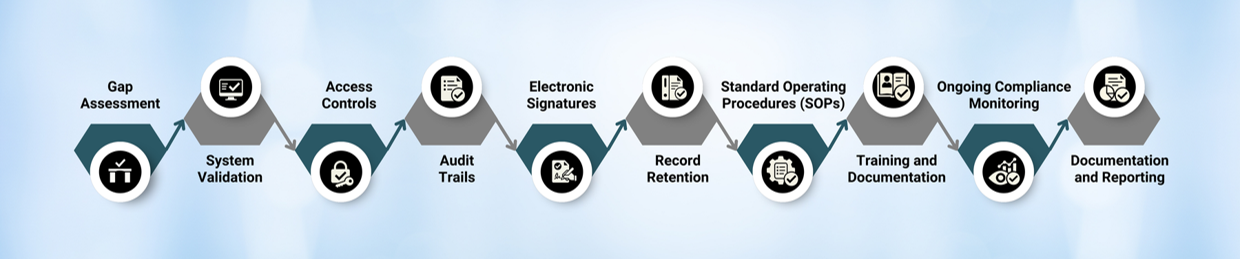
STEP FOR IMPLEMENTATION OF 21 CFR PART 11:
Services for ccit method setup:








.png)