Filter Integrity Tester: LT-5000 FLIT
“Integrity Assured, Intelligence Built In..!”
The LT-5000 FLIT Filter Integrity Tester is a compact, high-precision system designed for reliable filter integrity measurement with advanced security and automation features.
As per ASTM, FDA 21CFR Part11, USP 1207, NABL, CE, ISO 9001:2008, EMA ANNEX 11 guidelines
Key Points
- Operates from 50–9000 mbar
- Supports Bubble Point, Diffusion Flow, and Water Intrusion tests
- Includes Ethernet & USB communication
- Built-in password protection, authority control, and e-signature
- Equipped with an automatic sterilization control system
Technical Specifications:
| SPECIFICATIONS | MICRO LEAK DETECTION |
|---|---|
| Input power | 100 to 260 VAC, 50 Hz, 120W |
| Environmental Condition | Ambient Temperature: +5°C to +40°C Relative Humidity: 10 to 80% |
| Unit | m bar/kpa/pa |
| Test Scope | BP: 50 to 9000 mbar DF: up to 1000 ml/min WI: 0.01 to 150ml/min |
| Test Accuracy | 0.075% |
| Modes | Bubble Point Test, Diffusion Flow Test, Water Intrusion Test |
| Print Function | Compatible |
| Internal Memory | Stores upto 5 years of data |
| Test Report | In-built non-editable, printable PDF format |
| Display Screen | 7"/10" color touch screen - resistive/capacitive |
| Connectivity | Serial Ethernet & USB |
| Weight | 6.5 Kg |
Features
- Offers a wide range of integrity testing, compatible with all existing test methods.
- Integrated security framework offering password protection, permission control, hierarchical authority grading, and electronic signature support.
- Compatible with SAP, ERP communication with various protocols like Profinet, UDB, OPC etc.
- Dedicated development team for customization
Application
- Filter Integrity Testers play a vital role precisely in the food and pharmaceutical industries
- Product range includes:
- Capsule filter housings
- Industrial filter cartridges
- Membrane filtration systems
- Syringe filter
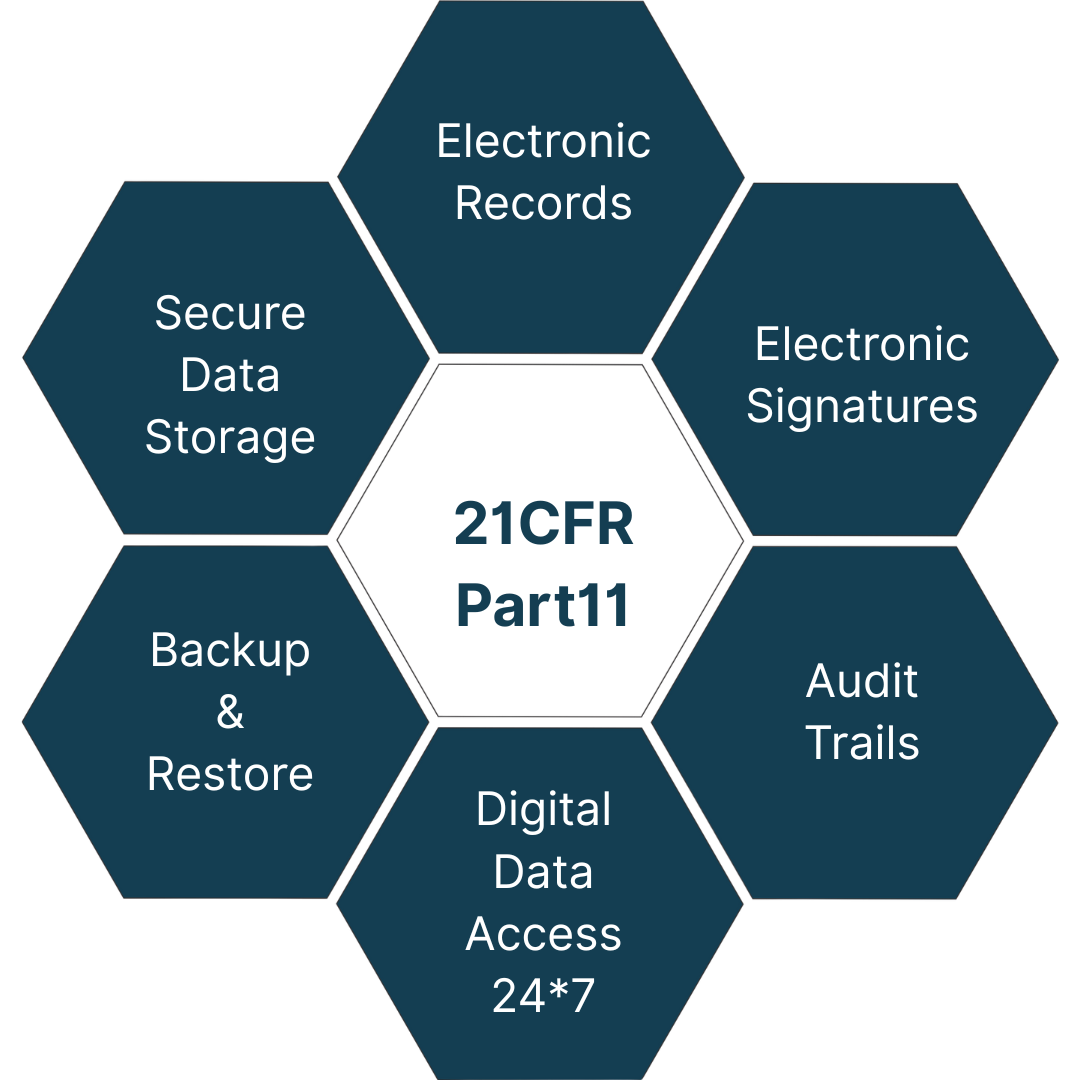
21 CFR PART 11 COMPLIANT LT-5000 FLIT
- To keep pace with the increasing use of electronic systems and technology in FDA-regulated industries, 21 CFR Part 11 was introduced, ensuring the same level of data integrity, authenticity, and reliability as paper-based systems.
- Basically, 21 CFR Part 11 is a regulation established by the U.S. Food and Drug Administration (FDA) that outlines the requirements for the use of electronic records and electronic signatures in FDA-regulated industries, including pharmaceuticals.
- Adhering to these guidelines, the introduction of Information Technology (IT) in compliance was a milestone.
- It establishes requirements for the security, integrity, and availability of electronic records and the validation of electronic systems used for GxP (Good Laboratory Practices, Good Clinical Practices, Good Manufacturing Practices) activities.
Audit Trail:
- Tamperproof & Non-Editable Audit Trail Data Format.
- The time stamp of the change of the parameter value & the user making the change.
- The audit trail records the following details:
- User Creation
- User Login/Logout
- Wrong attempts at login
- User Block / Unblock by Administrator
- Old value & new value of the parameter change
- The time stamp of each event
Electronic Signatures:
- Through 3/4 user level validation & authentication
System Data & Data Backup:
- HMI offers basic connectivity for data exchange with Central SCADA / MES / ERP by following the means:
- Through OPC UA
- Through Data file transfer
- Through USB / SD Card Backup
Electronic Data Record & Data Storage:
- Review of the reports on the HMI Screen for Production, Alarms, & Audit Trail.
- Storage limit can be interlocked in terms of the number of batches produced or % of the memory consumed.
User Management Functionalities:
- Password-protected individual Unique user account.
- Password Complexity.
- Minimum 8 Character password length.
- Configurable Number of Wrong Attempts.
- User Block/Unblock Facility.
- Password Validity.
- Multiple User Levels as per the User Rights.
Report Generation & Printing:
- Batch data is stored in a secure database format, & the Batch Report can be generated using this data.
- The report printing has been offered in multiple ways.
- Online printing of Alarms, Events, & Logged data through Serial Printer.
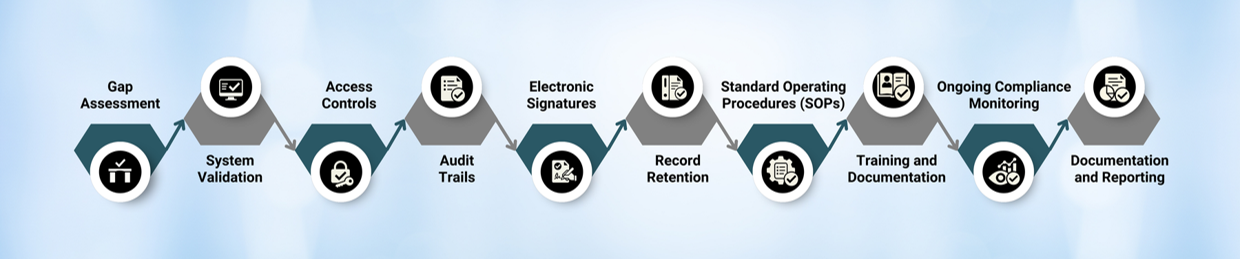
STEP FOR IMPLEMENTATION OF 21 CFR PART 11:
Services for FLIT method setup:
- PAN India service support
- Dedicated development team for customization
- High over-pressure sensor
- Accuracy to detect up to 0.5 µm
- Micro drilling services
- Micro-calibrated leak
- Master (solid dummy)
- Positive control (1µm, 5µm, 10µm, 20µm)
.png)